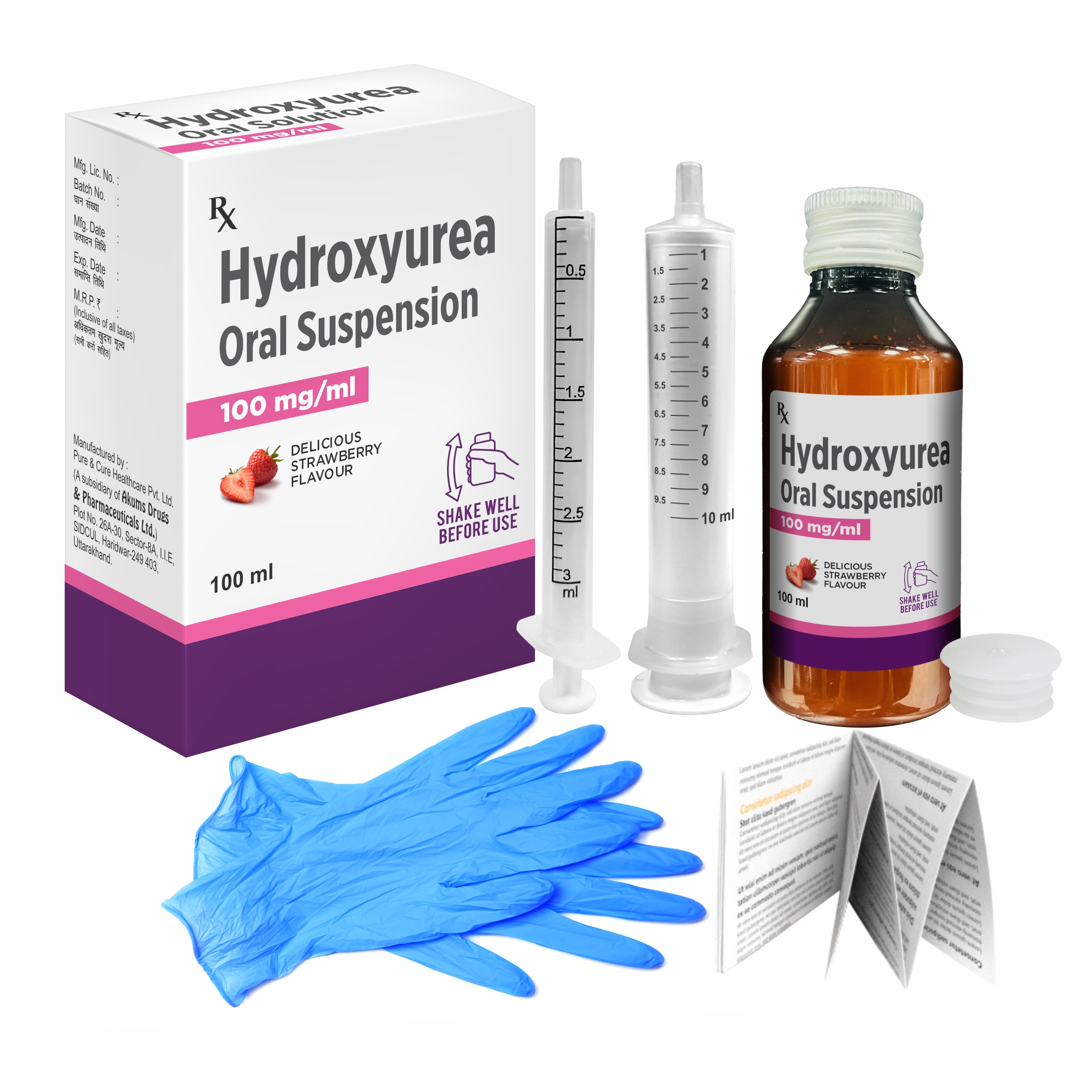
Akums Drugs and Pharmaceuticals, an India Focused Contract Development and Manufacturing Organizations (CDMO), was awarded a patent for its Room Temperature Stable Oral Suspension of Hydroxyurea, a breakthrough formulation aimed at managing Sickle Cell Disease (SCD). This development offers a significant advance in addressing the storage and accessibility challenges associated with Hydroxyurea solution.
In comparison to Hydroxy Urea Capsule (500mg), Akums Hydroxy Urea suspension offers dose flexibility based on body weight of the SCD Patients, thus offer significant advantage for pediatric and adolescent patients
Sickle Cell Disease, a genetic blood disorder, leads to severe health complications such as anemia, frequent pain episodes and other debilitating symptoms, affecting millions worldwide especially in India and Africa. According to the 2011 Census, 8.6% of India’s population is from tribal communities, many of whom are disproportionately affected by SCD.
Read more: Medtech innovations that make you look: Head transplants, smart pills, kidney transplant from pigs
Addressing this pressing public health issue, Akums’ new formulation offers a key advantage over traditional Hydroxyurea solution that require refrigeration between 2–8°C. The new oral suspension remains stable at room temperature, providing a practical solution for widespread distribution, particularly in the tribal areas with limited access to cold storage facilities.
Commenting on this milestone, Sanjeev Jain, Managing Director of Akums, said, “Our constant endeavor is to work on affordable medicines for Orphan Drugs and reduce dependency on imported medicines ensuring patient safety from rare diseases with timely and necessary treatment.”
Our innovative oral suspension remains stable at room temperature, ensuring greater accessibility and providing an efficient solution for widespread distribution, particularly in tribal areas where cold storage facilities are scarce — Sandeep Jain, Managing Director of Akums
Sandeep Jain, Managing Director of Akums, added, “This patent reflects our dedication to the ‘Make in India’ initiative and our commitment to offering affordable, high-quality pharmaceutical solutions as envisioned by the Government of India. We continue to prioritize innovation to make essential treatments accessible and cost-effective for all. Tribal communities make up 67.8 million people across various states in India. Many within these communities are disproportionately affected by Sickle Cell Disease (SCD). We are happy that our R&D scientists have provided a much required formulation that is suitable for all age groups, especially pediatric and adolescent patients suffering from SCD. Our innovative oral suspension remains stable at room temperature, ensuring greater accessibility and providing an efficient solution for widespread distribution, particularly in tribal areas where cold storage facilities are scarce.”
Read more: AI innovations helping healthcare
Akums’ breakthrough aligns with India’s National Sickle Cell Anemia Mission, spearheaded by Prime Minister Narendra Modi, which aims to enhance the quality of life for SCD patients by improving treatment options. In comparison to Hydroxy Urea Capsule (500mg), Akums Hydroxy Urea suspension offers dose flexibility based on body weight of the SCD Patients, thus offer significant advantage for pediatric and adolescent patients.